ghtf-sg2-n54r8-guidance-adverse-events-061130医疗器械上市后监测:医疗器械不良事件报告全球指南



摘要:
展开>>
收起<<
GHTF/SG2/N54R8:2006FINALDOCUMENTGlobalHarmonizationTaskForceTitle:MedicalDevicesPostMarketSurveillance:GlobalGuidanceforAdverseEventReportingforMedicalDevicesAuthoringGroup:StudyGroup2Date:30November2006GeorgetteLalis,GHTFChairThedocumenthereinwasproducedbytheGlobalHarmonizationTaskForce,whichiscomp...
声明:如果您的权利被侵害,请联系我们的进行举报。
相关推荐
-
IVD产品设计开发以及注册申报流程图VIP免费
 2024-04-12 148
2024-04-12 148 -
医疗器械设计开发控制指南VIP免费

 2024-04-12 220
2024-04-12 220 -
YY∕T 0664-2020医疗器械软件软件生存周期过程核查表VIP免费
 2024-04-12 202
2024-04-12 202 -
创新医疗器械注册申报流程

 2024-05-02 110
2024-05-02 110 -
20221028_医疗器械生产现场核查缺陷分析交流(江苏药省监局审核查验中心) (1)VIP免费

 2024-05-09 73
2024-05-09 73 -
医疗器械网络安全漏洞自评报告VIP专享

 2024-11-18 263
2024-11-18 263 -
内审检查表 MDR法规VIP免费
 2025-04-07 157
2025-04-07 157 -
国产三类医疗器械首次注册-申报前准备工作VIP免费
 2025-09-03 19
2025-09-03 19 -
国产三类医疗器械首次注册-申报流程VIP免费
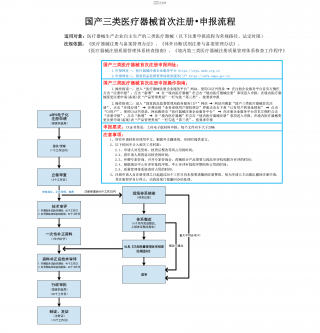
 2025-09-03 23
2025-09-03 23 -
国产三类医疗器械变更注册VIP免费
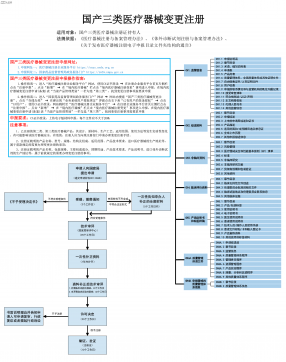
 2025-09-03 18
2025-09-03 18
作者:宁静致远
分类:专业资料
属性:37 页
大小:389.67KB
格式:PDF
时间:2025-04-16


