ISO TR 20416-2020 医疗设备-上市后制造商监督
Medicaldevices—Post-market
surveillanceformanufacturers
Dispositifsmédicaux—Surveillanceaprèsmisesurlemarché
incombantauxfabricants
©ISO2020
TECHNICAL
REPORT
ISO/TR
20416
Referencenumber
ISO/TR20416:2020(E)
Firstedition
2020-07
ISO/TR20416:2020(E)
ii©ISO2020–Allrightsreserved
COPYRIGHTPROTECTEDDOCUMENT
©ISO2020
Phone:+41227490111
Website:www.iso.org
ISO/TR20416:2020(E)
Foreword........................................................................................................................................................................................................................................iv
Introduction..................................................................................................................................................................................................................................v
1Scope................................................................................................................................................................................................................................. 1
2Normativereferences...................................................................................................................................................................................... 1
.....................................................................................................................................................................................1
4Purposeofpost-marketsurveillanceprocess....................................................................................................................... 2
5Planningofpost-marketsurveillance............................................................................................................................................ 3
........................................................................................................................................................................................................... 3
................................................................................................................... 4
.......................................................................................................... 5
.............................................................................................................................................. 7
......................................................................................................................................................................................... 7
......................................................................................................................................................................... 7
......................................................................................................................
...................................................................................................... 9
............................................................................................................................................................................................. 9
...................................................................................................................................................................................... 9
.................................................................. 9
........................................................................................................................................ 9
................................................................................................................................................................ 10
...............................................................................................................................................11
6Reviewofthepost-marketsurveillanceplan......................................................................................................................12
6.1Purposeofthereview.................................................................................................................................................................... 12
........................................................................................................................................................................................................ 12
6.3Review......................................................................................................................................................................................................... 13
AnnexAExamplesofdatasources.................................................................................................................................14
AnnexBExamplesofdataanalysismethods......................................................................................................25
AnnexCExamplesofpost-marketsurveillanceplans...............................................................................31
Bibliography.............................................................................................................................................................................................................................43
©ISO2020–Allrightsreservediii
Contents
摘要:
展开>>
收起<<
Medicaldevices—Post-marketsurveillanceformanufacturersDispositifsmédicaux—Surveillanceaprèsmisesurlemarchéincombantauxfabricants©ISO2020TECHNICALREPORTISO/TR20416ReferencenumberISO/TR20416:2020(E)Firstedition2020-07ISO/TR20416:2020(E)ii©ISO2020–AllrightsreservedCOPYRIGHTPROTECTEDDOCUMENT©ISO2020Phon...
声明:如果您的权利被侵害,请联系我们的进行举报。
相关推荐
-
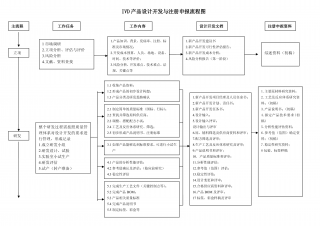
IVD产品设计开发以及注册申报流程图VIP免费
 2024-04-12 136
2024-04-12 136 -
医疗器械设计开发控制指南VIP免费

 2024-04-12 212
2024-04-12 212 -
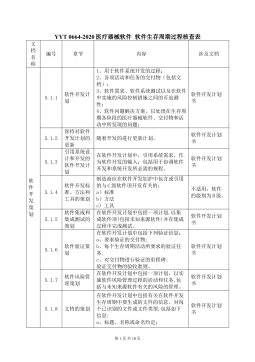
YY∕T 0664-2020医疗器械软件软件生存周期过程核查表VIP免费
 2024-04-12 189
2024-04-12 189 -
创新医疗器械注册申报流程

 2024-05-02 105
2024-05-02 105 -
20221028_医疗器械生产现场核查缺陷分析交流(江苏药省监局审核查验中心) (1)VIP免费

 2024-05-09 73
2024-05-09 73 -
医疗器械网络安全漏洞自评报告VIP专享

 2024-11-18 258
2024-11-18 258 -
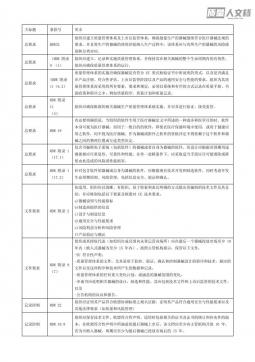
内审检查表 MDR法规VIP免费
 2025-04-07 147
2025-04-07 147 -
国产三类医疗器械首次注册-申报前准备工作VIP免费
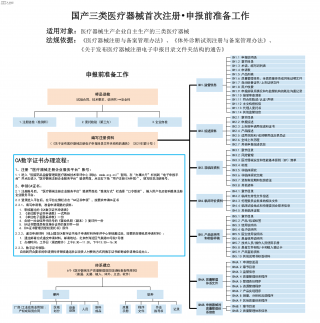
 2025-09-03 15
2025-09-03 15 -
国产三类医疗器械首次注册-申报流程VIP免费
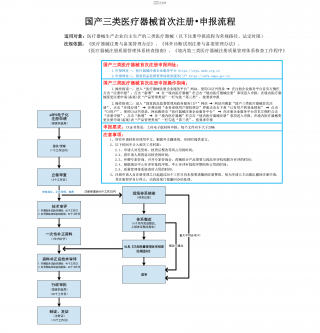
 2025-09-03 18
2025-09-03 18 -
国产三类医疗器械变更注册VIP免费
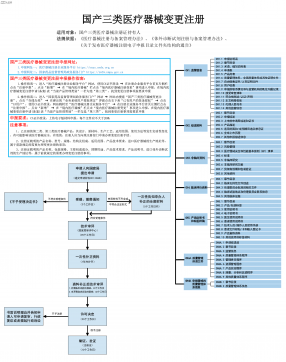
 2025-09-03 17
2025-09-03 17
作者:这个名字不太冷
分类:法规规范
属性:50 页
大小:3.12MB
格式:PDF
时间:2024-04-30